Dear Colleagues,
It is with great pleasure that I introduce you and your respective organisations to the Australian Medical Professionals Society. This email deals with several issues which are of concern to our membership and, we hope, yours. At the top of the list is the issue of medical free speech and its ramifications for true dialogue, debate, and informative patient interaction in Australia. Also, this email and the report of Dr Phillip Altman, makes available to you and your members a cutting edge update on the COVID-19 vaccinations and a comprehensive analysis of associated Adverse Events, together with implications for Australian practice. Finally, we draw your attention to our Health Reform Declaration, a statement which is gaining support as it highlights critical issues and potential solutions, within the complex environment of Australian Health Law.
Australian Health Professionals and Scientists have been actively discussing and contemplating the profound health measures undertaken within Australia over the last 2&1/2 years. However, we believe the current range of medical, medicolegal and medicopolitical issues brought about by the pandemic requires a greater breadth of discussion – not less – within and between our respective organisations and memberships.
One of the chief concerns of our membership is that of medical free speech. Contingent to a joint statement received from AHPRA and the National Boards on 9 March 20211, Australian Health Professionals numbering over 825,000 were essentially forbidden from publicly questioning the science underlying the emerging COVID-19 injectables, let alone questioning any government messaging urging Australians to be vaccinated because these products were deemed ‘safe and effective’. The effect of this unilateral action was to undermine professional independence and, in so doing, strip away years of training, academic achievements, qualifications, awards and expertise. However well intentioned, this gagging by bureaucratic decree inserted AHPRA and the National Boards between the Clinician and their Patient, in addition to counteracting normal robust interprofessional dialogue, as more data emerged.
Indeed, now 17 months later and after numerous forms of pressure to take up the COVID-19 injectables in various age categories, a tremendous amount of data is available to more fully and accurately inform clinicians about these products. This literature includes over one thousand2 peer reviewed studies reporting of the harms being seen around the world, up to December 2021. In addition, it has become clear that the risk of serious illness and death attributable to COVID-19 disease is heavily weighted to the elderly and those with known co-morbidities, while in contrast, younger Australians are relatively resistant. Also, since the advent of the Delta and Omicron variants, it is highly questionable whether the vaccines are preventing transmission or illness.
In any event, the implied and intended outcome of the gagging was to see Doctors and Health Professionals effectively mandated to support the government campaign to have the Australian population injected with drugs for which there was no adequate short-, medium-, or long-term safety or efficacy data. Indeed, the rush to market and Provisional Approval occurred despite the absence of the usual pre-clinical studies, including testing for Carcinogenicity and Genotoxicity. In this regard, it should be of serious interest that a peer-reviewed investigation3 has demonstrated that mRNA-derived Spike proteins enter the cell nucleus and interfere with DNA. However, many critical facts like these became forbidden subjects for Health Professionals and Doctors to raise with their patients, let alone in public forums. Thus, we contend that the joint statement of 9 March 2021 has compromised proper and informed consent in Australia.
Especially given the lack of available pre-clinical research for each of these products, or clinical studies powered to detect early safety signals at the time of Provisional Approval, the need for ongoing critical appraisal of pharmacovigilance data remains paramount, to instruct responsible day to day practice. To date, none of the makers of the COVID-19 injectables have been able to stringently show their products to be Safe or properly Effective. To date, Adverse Events flowing from these products are at historically unprecedented levels globally and continue to rise. And again, to date, no other drugs in human history have reported more deaths, illnesses, injuries, and disabilities, which number as follows (to 28 June 2022):
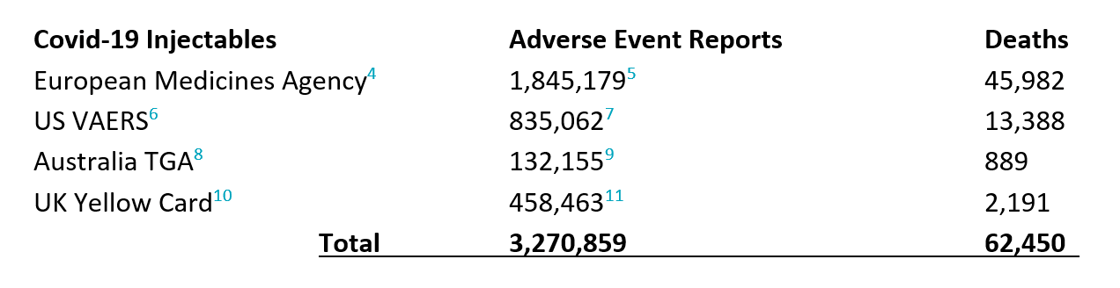
It is widely acknowledged that all Adverse Event reporting systems suffer from under-reporting12, an inherent challenge for passive reporting systems and their interpretation. For US VAERS reporting in respect of the COVID-19 injectables, the Under-Reporting Factor (URF) has been estimated to be between 40-49x13. If a conservative URF of 10x is applied, the above figures begin to more realistically represent the likely true effects of the Covid-19 injectables:

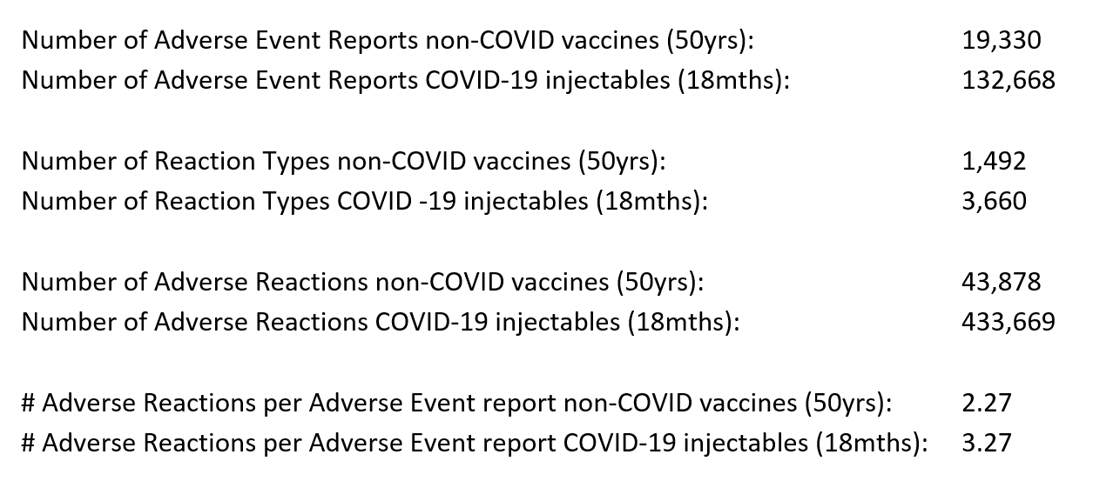
To be clear, the TGA has received more Adverse Event reports in 2021 through June 2022 for the COVID-19 vaccines, than they have been seen for all other vaccines in the preceding 50-year period. A similar explosion in Adverse Event reports for the COVID-19 injectables has occurred in all other countries that chose to deploy them14, but in Australia, comparing the period from 197115 until the start of 2021 in respect of traditional protein-based vaccines, to the period from 1 February 2021 through 8 June 2022 in respect of the COVID-19 injectables, we observe the following:
To assist your organisation and membership to understand the causes leading to these concerning signals, we provide to you the comprehensive and up-to-date report of Dr Phillip Altman. By way of background, Dr Altman’s report has been used in modified formats to assist judiciaries in Australia and New Zealand to understand the scientific evidence behind the COVID-19 injectables. We believe it is proving to be the long-awaited body of work needed by the Judicial, Medical, and Scientific communities of Australia, to bring clarity by critical scientific appraisal during these controversial times of COVID-19.
Since your organisation is now in possession of the information and resources contained in the linked report, we ask that your members also receive the same for the benefit of their being fully informed as to the state of the science surrounding COVID-19. After considerable consultation, AMPS is of the opinion that Australia is experiencing a highly significant iatrogenic event. Further, we believe that this did not have to occur: it could have been avoided, but for the state of Australia’s health law leading into the pandemic. AMPS is strenuously of the view that in order to avoid a repeat of the recent past, Australian health law requires urgent reform. To this end we invite every organisation receiving this email, including every parliamentarian CC’d, to review the Declaration and Urgent Demands for healthcare law reform set forth on the following page:
https://amps.redunion.com.au/healthreformdeclaration
On the above Declaration page is also found Proposed Amendments to the Health Practitioner Regulation National Law, and Proposed Amendments to the Therapeutic Goods Act.
Many organisations receiving this email have members who are directly affected by the overarching powers of AHPRA and the National Boards, who have tended to dictate rather than consult with their registered members. This has caused a dangerous interference with the provision of information, for the purpose of each Australian exercising their right to fully Informed Consent, while it has also unduly and harshly seen Health Professionals sanctioned for seeking to uphold ethics and their Codes of Conduct.
It is not only regarding COVID-19 that AHPRA has been perceived to show over-reaching powers. Dissatisfaction and fear of AHPRA is widespread amongst many health professionals as evidenced by the Victorian branch of the AMA calling for a Royal Commission16 into AHPRA’s conduct.
Equally, we say it is evident that Australians have suffered as a consequence of the Provisional Approval pathway laws. These have facilitated the rapid entry of significantly undertested products into the Australian market, despite their being recognised to be highly novel and experimental. Nonetheless, the COVID-19 injectables were mandated in many jurisdictions and workplaces, causing large numbers of Australians to feel coerced and simultaneously baffled by the inability of Doctors and other Health Professionals to give them a voice.
This can all be changed.
We implore you as fellows and colleagues to give the information and resource contained in this email your greatest attention, with a view to sharing the same with your members. There will doubtless be many questions arising from our email and we invite further discussion with you. All of your considerations and efforts towards the continued promotion of evidence-based medical science are greatly appreciated.
Yours sincerely,

Associate Professor Christopher Neil
MBBS, FRACP, PhD
Incoming President
Australian Medical Professionals Society
[1] https://www.ahpra.gov.au/News/2021-03-09-vaccination-statement.aspx
[2] https://www.covidmedicalnetwork.com/coronavirus-facts/vaccine/4_5902465845702954112.pdf
[3] https://www.mdpi.com/1467-3045/44/3/73/htm
[4] https://www.adrreports.eu/en/covid19_message.html – Pfizer, Moderna, AstraZeneca, Janssen
[5] Individual reports refer to a single patient, where more than one adverse reaction is often included.
[6] https://openvaers.com/covid-data (only US/Territories) – Pfizer, Moderna, AstraZeneca
[7] Individual reports refer to a single patient, where more than one adverse reaction is often included.
[8] https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-23-06-2022 – Pfizer, Moderna, AstraZeneca
[9] Individual reports refer to a single patient, where more than one adverse reaction is often included.
[10] https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting – Pfizer, Moderna, AstraZeneca
[11] Individual reports refer to a single patient, where more than one adverse reaction is often included. The 458,463 reports received to 24 June 2022 reported a total of 1,495,273 various forms of adverse reaction.
[12] https://scholar.google.com.au/scholar?hl=en&as_sdt=0%2C5&as_vis=1&q=EMA+ADR+under-reporting&btnG=
https://vaers.hhs.gov/data/dataguide.html
[13] https://stevekirsch.substack.com/p/latest-vaers-estimate-388000-americans
https://jessicar.substack.com/p/the-true-under-reporting-factor-urf
[14] https://worldcouncilforhealth.org/resources/covid-19-vaccine-pharmacovigilance-report/
[15] See DAEN website for no. of adverse events non-COVID vaccines and Covid injectables.
[16] https://insightplus.mja.com.au/2022/27/ama-victoria-to-call-for-royal-commission-into-ahpra/
Source – https://amps.redunion.com.au/covid19_evidence_based_information
