Also, AstraZeneca has been withdrawn completely
In its most recent Covid booster advice, the Australian Technical Advisory Group on Immunisation (ATAGI) finally acknowledges that for healthy young people, the risks may outweigh the benefit:
”Adolescents and younger adults have a lower age-related risk of severe COVID-19, and a comparatively higher risk of myocarditis following vaccination.”

Source: ATAGI 2023 booster advice
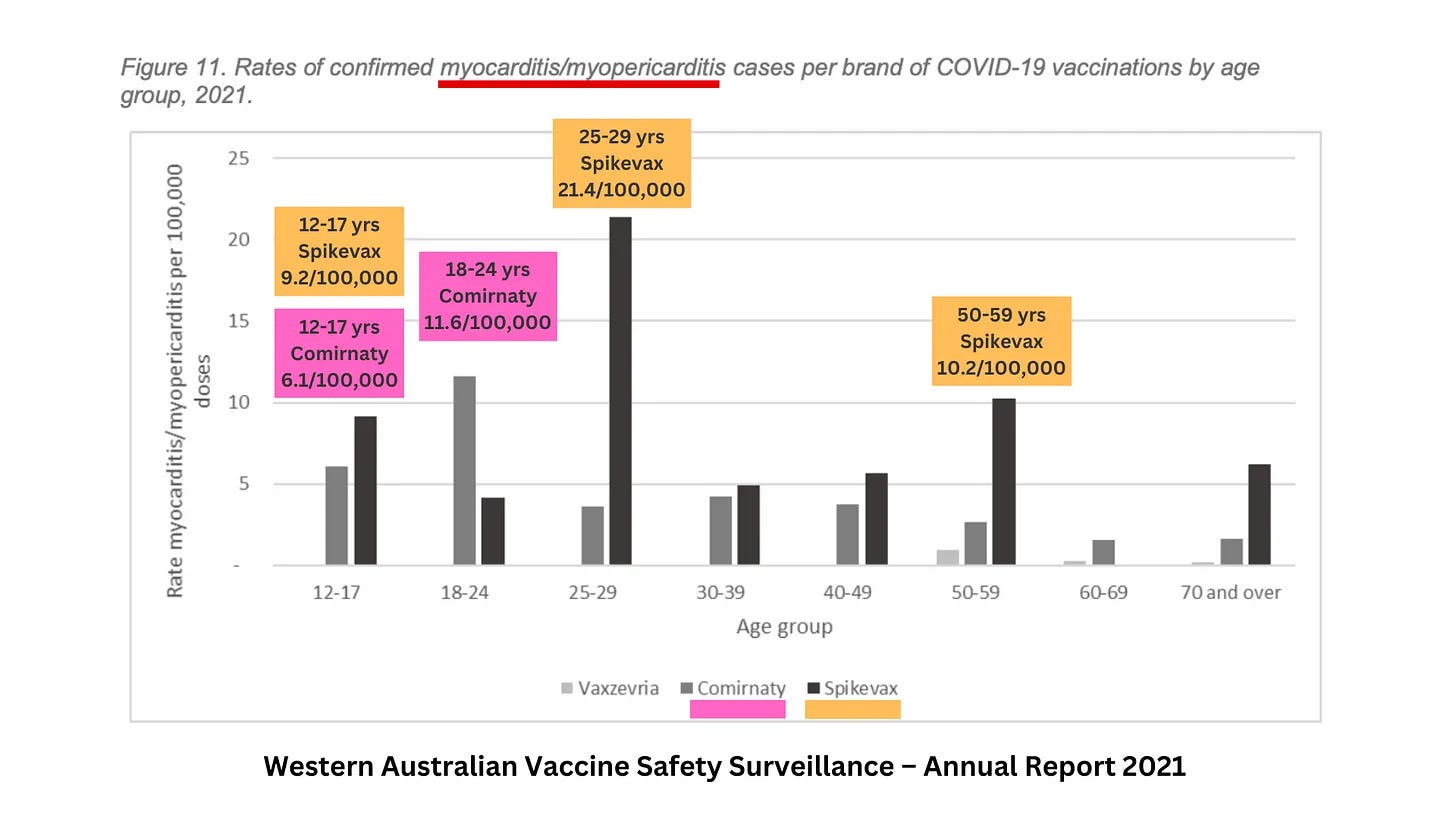
This was evident in the WA vaccine safety data that I reported on last week, in which the highest rates of myocarditis (and myopericarditis) are clustered around the youngest age groups.
Additionally, ATAGI acknowledges that Covid doesn’t pose much threat to young people, which respected experts have only been saying for about three years now.
This makes the risk/benefit analysis of Covid vaccines for young people tip more to the risk end of the scale.
Safe and effective? Now it depends on your unique patient profile.
For people under 65 years of age, ATAGI recommends that the decision to boost (or not) should, “take into account an individual’s age, risk factors for severe COVID-19, number and timing of previous doses or previous infection, and risk factors (predominantly age) for myocarditis and pericarditis following vaccination.” The notion of getting personalised vaccination advice for one’s unique medical situation is such a departure from the universal blanket vaccination rules of the past two years, I had to re-read it to make sure it was real.

Source: ATAGI Booster Advice, 08 Feb 2023
The recommended age for healthy people receiving boosters has also been bumped up from 50 to 65 and over. Have a look again at that myocarditis chart from the WA vaccine safety data above – specifically, the 50-59 age bracket.
AstraZeneca vaccine no longer available
As of Monday 20 March, AstraZeneca’s Vaxzevria vaccine is no longer available in Australia.
Source: Vaxzevria (AstraZeneca)
At first I thought, the TGA must have finally pulled the pin after the product killed 13 Australians (the officially acknowledged number of fatalities). The WA Safety report also showed confirmed cases of thrombosis, anaphylaxis and Guillain Barre following vaccination with Vaxzevria. The TGA must have read the report and taken action.
But no.
A spokesperson for the Department of Health advises that in fact it was AstraZeneca who decided to discontinue the product in Australia, not the TGA, and that Vaxzevria remains provisionally approved. AstraZeneca has been contacted for comment and this post will be updated accordingly if a reply is received.
Meanwhile, last month the TGA recalled a class of cough medicines from the shelves for its potential to cause anaphylaxis when used in conjunction with undergoing general anaesthetic.

Source: TGA media release 28 February 2023
The TGA states, “Up to 9 February this year, the TGA has also received 50 reports of Australian cases of suspected pholcodine-related anaphylactic reactions to neuromuscular blockers, including one fatality.”
One fatality is enough to pull a cough syrup. 13 fatalities following a vaccine? Sacrifice to a holy cow.
Finally, for overseas readers, don’t think that just because ATAGI has changed its recommendations that the media and health officials are running PSAs warning young people of the risks associated with boosters.
Source – https://rebekahbarnett.substack.com/p/high-risk-low-benefit-of-covid-boosters
