Facebook requested talking points from the Biden administration to “get ahead” of the possibility that people might be less likely to get a COVID-19 vaccine after evidence emerged that the Johnson and Johnson (J&J) single-dose vaccine might cause life-threatening blood clots in some rare cases, documents shared with the Daily Caller News Foundation show.
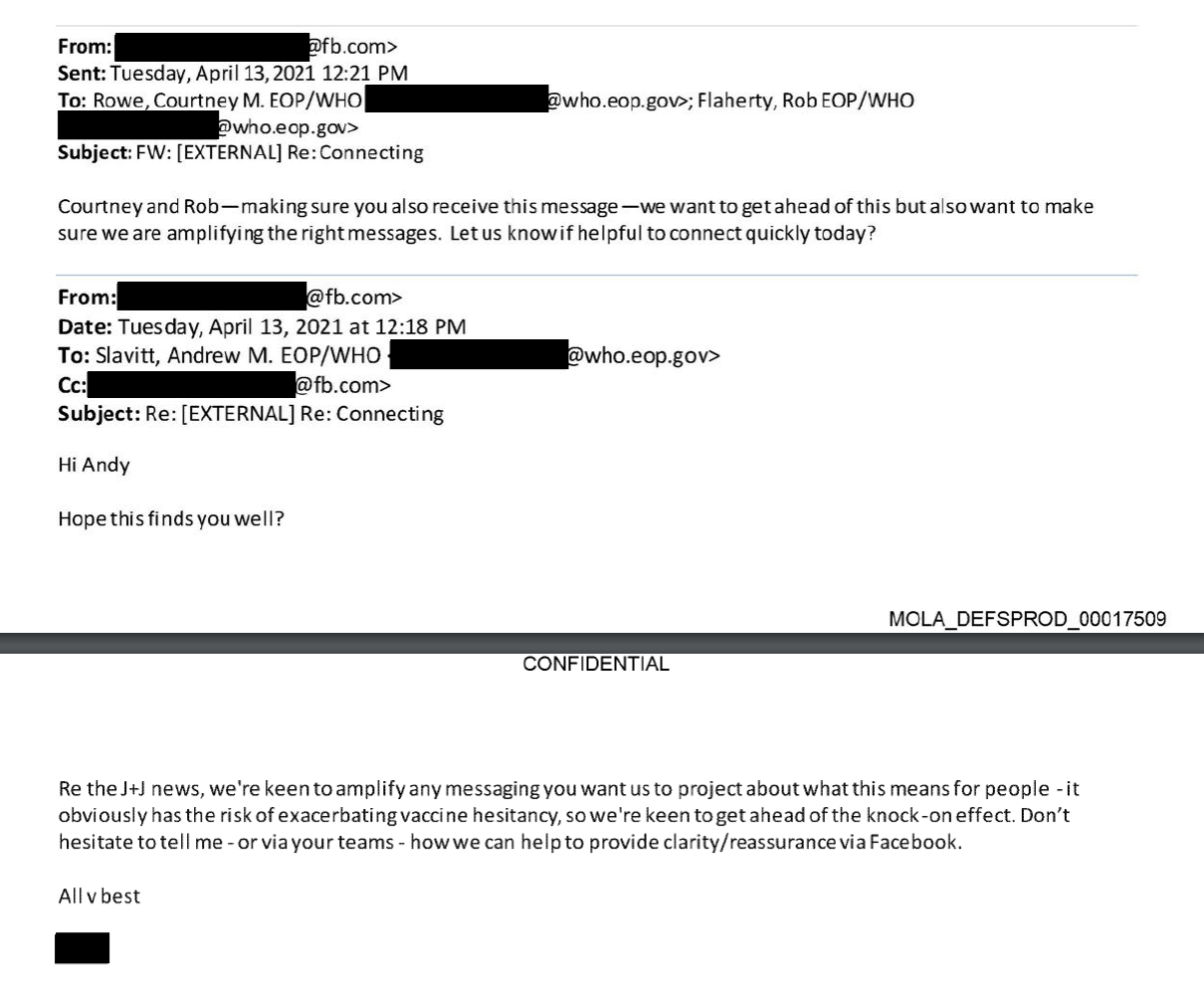
The U.S. Centers For Disease Control (CDC) and Food and Drug Administration (FDA) issued a joint statement on April 13, 2021, issuing a pause on the use of the J&J COVID-19 vaccine after they identified 6 cases where adult women — out of roughly 7 million individuals who receive the dose — developed severe blood clots. A Facebook employee wrote an email later that day asking Biden administration COVID-19 czar Andrew Slavitt for “any messaging” that the White House might want the company to promote, looping in White House Director of Digital Strategy Rob Flaherty and Director of Strategic Communications and Engagement for the COVID-19 Response Team Courtney Rowe in a follow-up email moments later, according to documents obtained by the New Civil Liberties Alliance (NCLA) and shared with the DCNF.
The NCLA is party to an ongoing lawsuit filed by the Attorneys General of Louisiana and Missouri alleging improper collusion between the federal government and social media companies, and obtained the emails via discovery.
“Re the J+J news, we’re keen to amplify any messaging you want us to project about what this means for people – it obviously has the risk of exacerbating vaccine hesitancy, so we’re keen to get ahead of the knock-on effect,” the employee wrote. “Don’t hesitate to tell me – or via your teams – how we can help you provide clarity/reassurance via Facebook.”
Flaherty responded to the email suggesting that the company create a context panel for information about the vaccine, suggesting that the company tell users that the clotting incidents “are very rare,” that the FDA and CDC are working on a treatment plan and that the vaccine is very different from the Moderna or Pfizer vaccines and that they were unaffected, according to the documents. The White House official would go on to say he was “happy to provide” more specifics about what the information in the context panel “should be.”
In addition, he provided Facebook with a statement from the White House, and informed the company that the CDC would be putting together a FAQ sheet on the J&J vaccine that the White House would “love to have amplified in whatever way possible,” according to the emails. Finally, Flaherty asked that Facebook commit to spreading as much positive news about the vaccine as negative, even going so far as to request that the company alter its algorithms to do so.
Flaherty believed that a “commitment from [Facebook] to make sure that a favorable review reaches as many people as the pause, either through hard product interventions or algorithmic amplification,” would be helpful to prevent “misinformation” surrounding the J&J vaccine, according to the emails.

White House Director of Digital Strategy Rob Flaherty responds to a Facebook employee requesting guidance on how to respond to the CDC and FDA’s decision to pause use of the Johnson & Johnson single-dose COVID-19 vaccine, April 13, 2021. Screenshot/New Civil Liberties Alliance
The Facebook employee thanked Flaherty “very much” for suggesting a context panel and asked if he had any ideas for how to update the company’s existing COVID-19 news panel, according to the emails. The employee also thanked Flaherty for his willingness to provide the CDC messaging, before promising that the company would “love to talk” about possibly modifying its algorithm to alter how information is shared and concluding with a promise to share data relating to “misinformation.”
Flaherty would spar with the company over the next week, seemingly over its refusal to censor Tucker Carlson’s criticism of the J&J vaccine and the administration’s vaccine messaging. The FDA ultimately restricted the use of the J&J vaccine to those who refused or were ineligible to take other vaccines, citing the risks associated with blood clots, in a statement issued May 5, 2022.
