In Denmark, 4.2 percent of Pfizer COVID-19 vaccine batches accounted for 71 percent of suspected adverse events (SAEs), according to Danish researchers in a recent study published in the European Journal of Clinical Investigation on March 30.
The study has raised serious concerns about the inconsistencies in the quality of different vaccine batches and the implications for vaccine recipients.
The Study
Danish researchers studied the rates of SAEs between different batches of the Pfizer-BioNTech vaccine, BNT162b2, which was administered in Denmark from December 27, 2020–January 11, 2022.
There were approximately 7.8 million doses administered to 3.7 million people from 52 different Pfizer vaccine batches during that time period.
SAEs of 43,496 were reported by 13,635 individuals, which was an average of 3.19 events per person.
“Unexpectedly, rates of SAEs per 1000 doses varied considerably between vaccine batches,” the researchers wrote in the publication.
They further noted these results were unexpected, since in the European Union vaccine vials and individual batch and dose uniformity are monitored “with stringent quality control” under the Official Control Authority Batch Release guidelines.
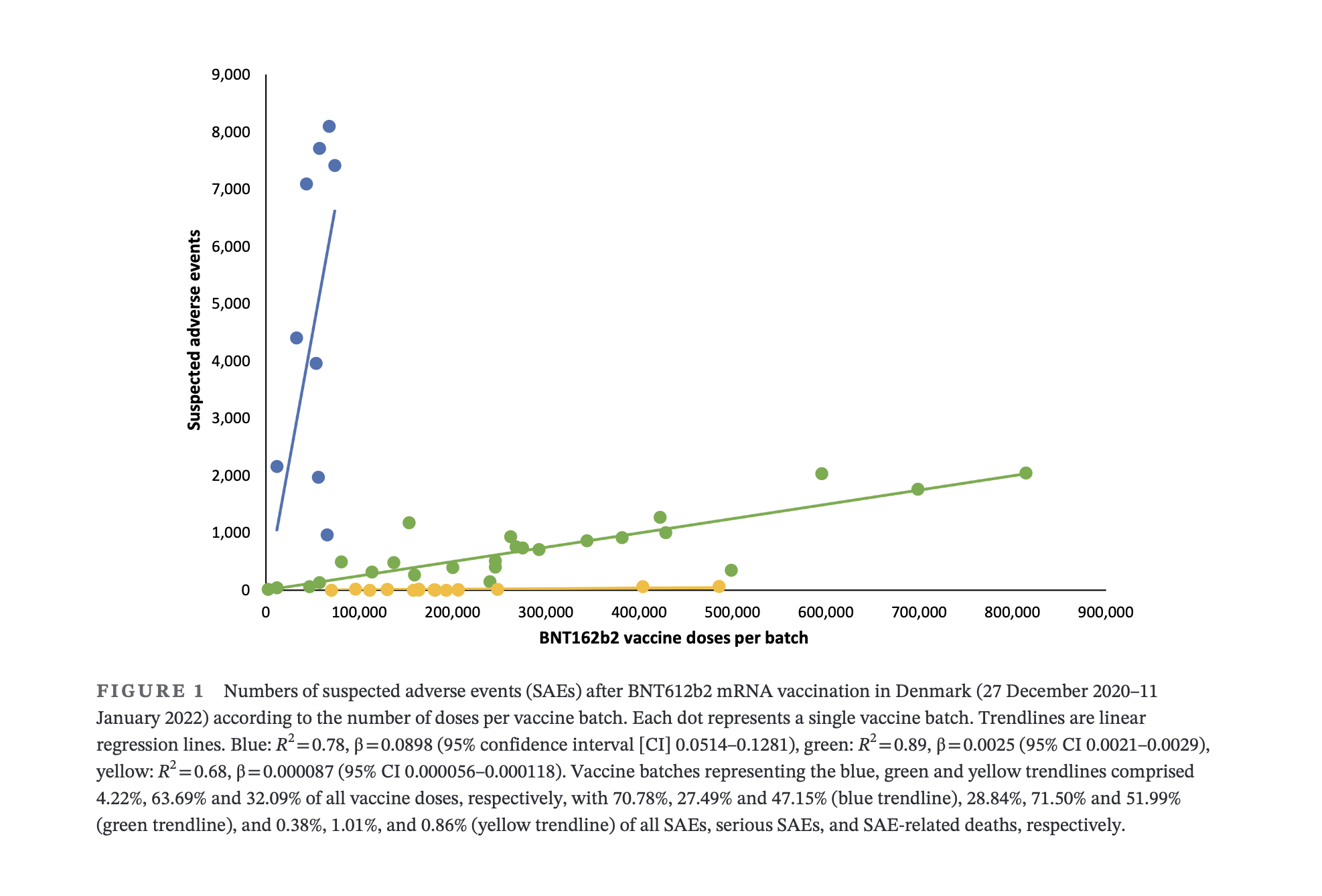
The blue trend line in the chart above shows that of the 71 percent of SAEs reported from only 4.2 percent of all vaccine doses, 27 percent were considered serious, and 47 percent resulted in death.
Serious SAEs mean hospitalization, life-threatening illness, or permanent disability.
“These are critically important results,” said Dr. Peter McCullough on Substack. McCullough is a renowned internist, cardiologist, and epidemiologist, as well as a contributor to The Epoch Times.
“They imply the COVID-19 vaccine debacle is indeed a product problem and not due to patient susceptibility in most circumstances,” McCullough said.
Jessica Rose, a Canadian molecular biologist who has focused on analyzing the Vaccine Adverse Event Reporting System (VAERS) data, applauded the authors for providing this evidence, yet pointed out the reporting could have been more accurate.
Since the doses deemed to be administered might not have been injected into people, Rose suggested the next important step is to examine the contents of the vials directly.
Max Schmeling, one of the research authors replied to Rose saying the data they obtained from the Danish Serum Institute “comes closest to being the real number of administered doses.” He further explained that this is because the shipped doses excluded any vials the Serum Institute may have already had in stock. However, after further investigation, Schmeling told The Epoch Times that a recent query of the Serum Institute verified that the number of shipped vaccine doses were essentially the same as the number of administered vaccine doses.
Questionable Vaccine Quality
One study found that vaccine vials are not of equal quality across batches. Researchers suggested the individual vaccine vial should be considered when investigating serious adverse reactions such as anaphylaxis since it might be caused by a defective vial.
Researcher Craig Paardekooper, on his website HowBadIsMyBatch.com believes that batches are in fact different, and provides evidence showing that the number and severity of adverse events vary across different batch numbers.
This problem was not found only in Pfizer’s COVID-19 vaccines.
In 2021, after 39 vials were found to contain foreign materials, three lots of the Moderna vaccine totaling more than 1.6 million doses were recalled in Japan.
In April 2022, Moderna recalled 764,900 doses of its COVID-19 vaccine in Europe after contaminants were discovered in a vial.
Many factors contribute to variations in vaccine quality, including vaccine manufacturing, storage, transportation, and clinical handling.
In the United States, the Food and Drug Administration has issued guidance for COVID-19 vaccine development and licensing. But according to McCullough, there are no inspections of the final filled and finished vials required under Emergency Use Authorization.
“The lack of inspections has led to a safety disaster. Some unfortunate patients are getting too much mRNA, contaminants, or both, and thus are exposed to damaging and in some cases, lethal injections,” said McCullough.
Study Limitations
The authors of the Danish study acknowledged there are certain limitations to their study.
The SAE reporting system managed by the Danish Medical Agency is a passive surveillance system similar to VAERS in the United States, so it may be under-reporting, over-reporting, or incomplete.
Additionally, the SAE case history of prior COVID-19 was unknown. Specific SAE types, demographics of SAE cases, and other factors were not examined.
“More studies are warranted to explore this preliminary observation and its consequences,” wrote the authors in the Danish report.
