The study, commissioned by the MOH, also indicates new adverse events not reported in Pfizer’s leaflet, and that some adverse events last more than a year. Despite being aware of these findings, the MOH is hiding them from the public and has recently authorized the booster dose for ages 5-11 and is preparing to approve the vaccine for infants

The serious findings revealed :
- Adverse events are 2-4 times more common among ages 5-11! This is despite the fact that immunization rates at younger ages are 3-4 times lower than older children.
- New adverse events that were not reported in Pfizer’s leaflet were identified.
- Contrary to what has been claimed, various adverse events, including serious ones, are not short-lived and transient. Some last more than a year.
- A significant proportion of the adverse events did not disappear by the end of the study, so it is not possible to know how long they lasted.
- Recurrence/exacerbation of adverse events following another dose was identified
While Pfizer CEO Albert Bourla is hosted in Israel and is promoting his company’s COVID vaccine for infants ahead of the Israeli Ministry of Health’s expected approval for infants in the coming days, it turns out that the ministry is hiding very serious findings on the vaccine’s adverse events in children, especially concerning children aged 5-11. Although the findings of the study, which was commissioned by the ministry itself, were presented to the Epidemiology Division three weeks ago, and despite their seriousness, the MOH authorized last week a third dose (“booster”) for ages 5-11 and announced that it plans to approve the vaccine for infants as early as this week.
In an urgent letter sent on Monday (June 27, 2022) by the Professional Ethics Front to the State Comptroller of Israel, Dr. Matanyahu Engelman, it was revealed that the findings of a study conducted by Prof. Matti Berkowitz, director of the Clinical Pharmacology and Toxicology Unit at Assaf Harofeh Hospital (Shamir) were presented to the Department of Epidemiology in early June. The study, which was commissioned by the Ministry of Health, examined the adverse events of Pfizer’s COVID vaccine for about six months (From December 9, 2021, until the end of May 2022). The study was based on an analysis of reports recorded in the Nahlieli system (a system that constitutes the Israeli national vaccine database, including the management of the accompanying information, which includes reports of adverse events and safety demonstrations). The letter to the State Comptroller stated that “The findings have been brought to our attention, and they are serious and indicate a risk to children, and in particular to young children aged 5-11”.
Today (June 29, 2022), the association sent another letter to the State Comptroller, detailing the serious findings, accompanied by slides presented to the Epidemiology Division by the research team led by Prof. Berkowitz.
The findings show, among other things, that adverse events are 2-4 times more common among young children aged 5-11; that there are new adverse events that have not been reported in Pfizer’s COVID vaccine leaflet; that contrary to what has been claimed, various adverse events, including neurological effects and menstrual disorders, are not short-term events that pass within a few days, but in many cases, they last for months or even more than a year. Moreover, the study found that a significant proportion of the symptoms did not disappear by the end of the study, so it is not possible to know how long they lasted; and a considerable part of the cases even if the adverse event ceased, it recurred after the next dose.
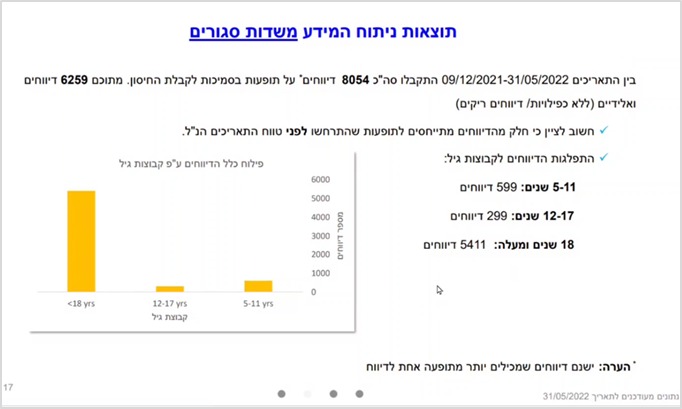
During the study period, 8,054 reports of vaccine-related adverse events were received in all age groups (5-11; 12-17; 18 and over), of which 6,259 were defined as “valid” (without duplication or empty reports ). In addition, 2,075 adverse events were reported as free text. Of the reports submitted as free text – five categories together constitute about 70% of all reported adverse events. These categories were analyzed by the research team, and include (in order of frequency): 1. Neurological effects; 2. General effects (not included in any of the categories specifically); 3. Menstrual disorders; 4. Symptoms in the musculoskeletal system; 5. Symptoms in the gastrointestinal tract/kidneys and urinary tract. Cardiovascular symptoms were noted in the sixth category, with slightly fewer reports compared to the fifth category. However, this category was not analyzed by the team, although it was precisely these adverse events, such as myocarditis and pericarditis, that were found to be related to the vaccine and common among teenagers and young adults. Also, according to the team members who presented the study, there are other symptoms such as autoimmune symptoms, which are important to analyze in order to better identify the safety profile of the vaccine, but at this stage have not been analyzed.
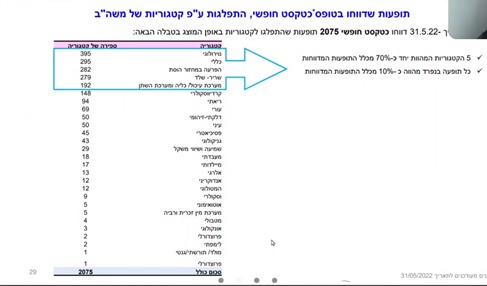
Adverse events are 2-4 times more common among ages 5-11
The researchers found that in the youngest age group – ages 5-11, adverse events are 2-4 times more common (depending on the specific adverse event described) compared to the older age group, aged 12-17. This finding is very surprising and especially troubling in light of the fact that the percentage of vaccinated among the young group is much lower compared to the larger age group. In fact, according to the MOH’s COVID dashboard, only 25.1% of children aged 5-11 received one dose of the COVID vaccine, 17.7% received two doses, and 0.1% received three doses. In contrast, among 12-15-year-olds 65.6%, 54.6% received two doses, and 14.8% received three doses. Among 16-19-year-olds 89.3% received one dose, 78.2% received two doses, and 45.9% received three doses. That is, while the immunization rate among ages 5-11 is 3-4 times lower for the first and second doses of the vaccine, the rate of reported adverse events in this group is 2-4 times higher!
It should be noted that the findings surprised even the research team itself, and were emphasized by them several times during the presentation.
The finding of a higher rate of adverse events among ages 5-11 compared with ages 12-17 emerges from both the closed fields and open text in Berkovitch’s analysis, and in almost all categories analyzed. In the closed fields it was found that 599 reports are among ages 5-11, while 299 reports are among ages 12-17 – that is, double the number of reports among the younger group.
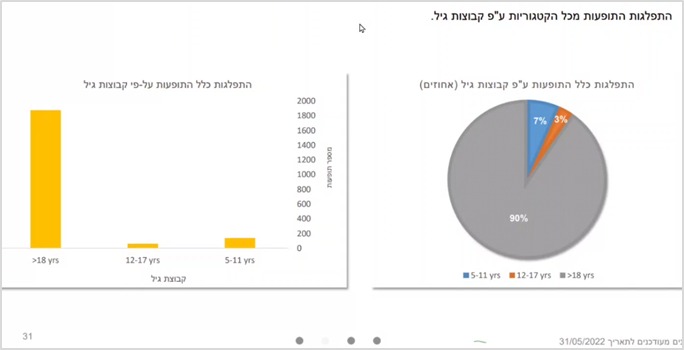
Of the adverse events reported as free text – 7% are among ages 5-11, and 3% among ages 12-17. In other words, the rate of adverse events reported among the younger children is almost 2.5 times higher than that of the older group.
For example, in the category of neurological adverse events – 6% are among ages 5-11, and 3% among ages 12-17. That is, the rate of neurological symptoms in younger children is twice as high as in the older group.
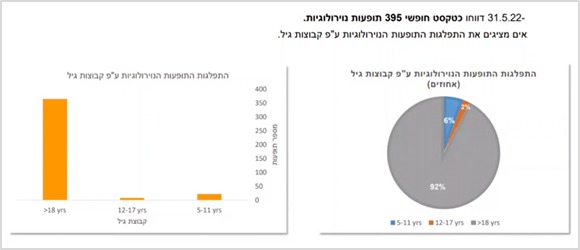
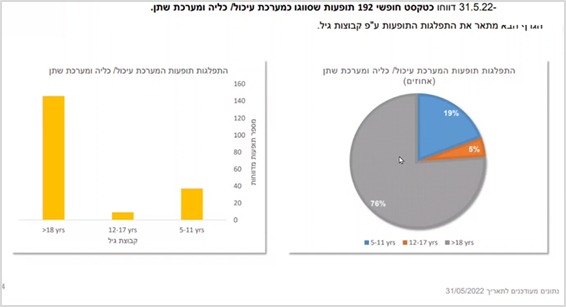
In the category of adverse events relate to the gastrointestinal / kidney and urinary system – 19% are among ages 5-11, and 5% are among ages 12-17. That is, the rate of reports in the younger group is 4 times higher than in the older group!
New adverse events that were not reported in Pfizer’s leaflet were identified

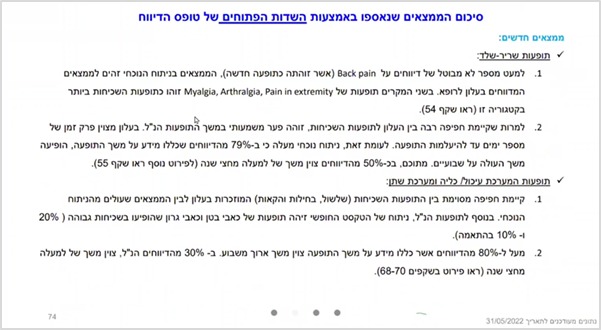
As can be seen from the slide below, the team identified and characterized neurological symptoms that were not previously known and are not mentioned in the physician’s leaflet of Pfizer’s Comirnaty vaccine, including Hypoesthesia (partial or complete decrease in skin sensitivity), Paraesthesia (abnormal skin sensation such as numbness, tingling, stinging or burning), tinnitus, dizziness and more.
Are the adverse events indeed short-lived and transient? Some last more than a year
The findings also include evidence that contrary to what has been claimed so far, various adverse events, including some serious ones, are not short-lived and pass within a few days, but in many cases last for months and even more than a year. It should be noted that the research team made it clear to the Department of Epidemiology that Pfizer needed to be notified regarding the long-term adverse events identified, as the company’s representatives said in a discussion held a few months ago that they had no knowledge of long-term adverse events.
Thus, as can be learned from the slide presented above, as well as from the following slide, the research team found that while studies examining the topic of menstrual disorders have so far claimed that such disorders are short-lived (up to several days), in the present study most reports indicate long-term adverse events. For example, more than 90% of the reports, which relate to menstrual disorders and specify characteristics of the duration of the problem, indicate long-term changes. Over 60% indicate menstrual disorders lasting more than three months: in 26% of the cases, the menstrual disorder lasted 3-6 months; In 15% of the cases it lasted 6-9 months; In 10% of them it lasted a whole year, and in another 10% it lasted over a year. The researchers also noted that in 30% of cases, the menstrual disorder was described as persistent. That is, it did not end at the time the report was delivered. Similarly, with regard to neurological adverse events, in about 68% of the reports which included a characteristic of the duration, the problem lasting more than a month were documented, of which 88% lasted more than three months.
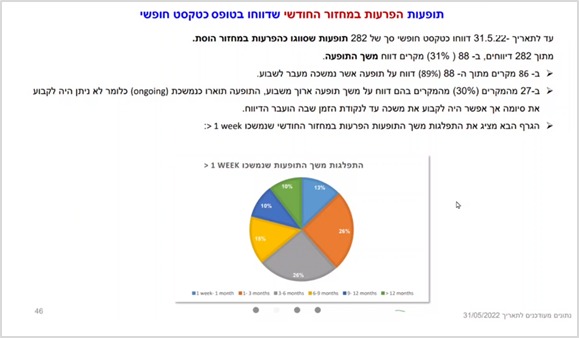
Similarly, other reported adverse events have also been identified as long-lasting. For example, with regard to musculoskeletal symptoms, the researchers wrote that while Pfizer’s’ leaflet stated that adverse events such as back pain only lasted for several days and then disappear, in the current analysis – in 79% of the reports which included information about these adverse events, it was noted that the duration lasted more than a week. Of these, about 50% indicated a duration of over six months!
Also with respect to adverse events related to the gastrointestinal, renal, and urinary tract, it was found that over 80% of the reports that included information on the duration of the problem indicated a duration longer than a week. In 30% of them, a duration of more than half a year was specified!
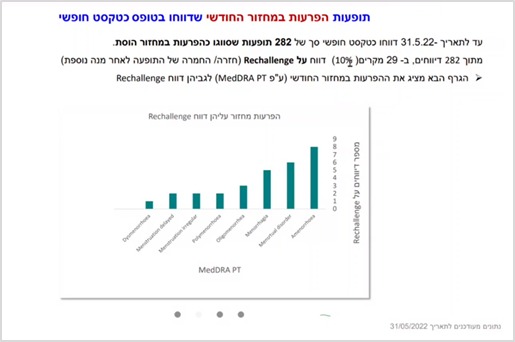
Recurrence/exacerbation of adverse events following a repeated dose
Another finding identified and characterized by the research team is a phenomenon called Rechallenge – recurrence /exacerbation of a previous adverse event after receiving another dose of the vaccine – thus, it was found that in 10% of cases where menstrual disorders were reported, there was a recurrence /exacerbation of the adverse events following another dose of the vaccine.
The two biggest HMOs “are keeping the information close to their chests”
The disturbing findings of this study should be regarded as even more serious in light of the fact that the study was based mainly on reports received from the small funds, and mainly from Meuchedet, as the research team said during the presentation. According to Prof. Berkowitz, the largest HMOs – Clalit HMO and Maccabi HMO, “are keeping the information close to their chests”. It follows that the analysis conducted is extremely lacking and limited, and its findings reflect a severe underestimation of the extent of the true side effects. “It is not clear how, when it comes to such a critical study, the major HMOs do not cooperate, and it is even more unclear why the Ministry of Health does not require them to transfer data to the study,” the association’s members wrote to the state comptroller.
It should be noted that even if the study was conducted on the basis of a full report from all the HMOs, these serious findings would still constitute under-reported, since the Nahlieli system is a voluntary reporting system, and it is known from the research literature that reporting in such systems only represents about 1% -10% of the adverse events found in reality. All the more so when it is known that the two central health funds did not cooperate in transmitting the data.
The concealment of the findings by the Israeli MOH could also have a serious and critical significance in relation to children and infant vaccination at a global level since Israel is considered the “laboratory state”, as claimed by Pfizer’s CEO, Dr. Bourla. The CDC and the FDA rely on the Israeli data and findings to base crucial decisions in the US – decisions which other health authorities globally later follow. Every decision made in Israel regarding children’s vaccination thus has a worldwide impact, and if the decisions are made while concealing and ignoring findings, the results can be disastrous.
Flashing warning lights – Ministry of Health ignores and hides
It turns out that the findings of the study conducted by Prof. Berkowitz and his team are not the first warning signal regarding the safety of Pfizers’ COVID vaccine in children. Active monitoring for adverse events was conducted by the HMOs in Israel for about four months among 172 children aged 5-11, who were vaccinated as an initial group outside the label (under the authorization of the vaccine for kids 12-15 years old), also demonstrated acute safety signals (Ministry of Health circular: “Vaccination of kids aged 5-11 years against the new Coronavirus – an exception for individual cases from 27.7.21”, reference 548562821). Another flashing warning light rises from the gap found among vaccinated kids aged 5-11 years old between the number of those who received the first dose and the number of those who received the second dose. According to data from the Ministry of Health, there is a gap of 92,000 children who did not return to receive the second dose of the vaccine. “This figure, published without any details or explanation, reinforces the warning signal regarding the safety of these vaccines”, the letter to the State Comptroller stated.
As mentioned, the findings of Prof. Berkowitz’s study were presented to members of the Epidemiology Division as early as the beginning of June. However, despite this information, the Epidemiology Division issued on June 14, 2022, a directive to administer a booster dose to children aged 5-11 (Division of the Epidemiology Division: “The new Coronavirus vaccine – a third dose for children aged 5-11 years – Update No. 8 of the 14.6.22 “, reference 548562821).
Moreover, last week the Ministry of Health’s epidemic treatment team discussed the approval of the vaccine for babies and toddlers aged six months to 5 years old in Israel. Contrary to the discussion in which the vaccine was authorized for the 5-11 years old children, the discussion on vaccinating the babies – the most vulnerable group – was not held in public. The only publicly available information about the course of the discussion came out from tweets by some health reporters, according to which Pfizer and Moderna representatives presented during the discussion and overview of the effectiveness of their vaccines in children. As far as is known, no independent experts were invited to the expected sequel discussion this week.
Given the serious findings of Prof. Berkowitz’s study, as well as the fact that the Ministry of Health did not disclose these findings, despite the time that has passed since they were presented to its Department of Epidemiology, and instead decided to approve the vaccine for ages 5-11, and due to the concern that the findings of the study will also be ignored in the upcoming discussion regarding the infant vaccine approval, an urgent Freedom of Information Request (FOIA) has been recently filed by the Professional Ethics Front (Application No. 657228). According to the request, the information should be disclosed even if it is still raw, “Out of fear that there is a blatant violation of parents’ right to informed consent, and because it constitutes gross negligence, and puts children and infants at risk”. However, so far no response has been received from the Ministry of Health.
In light of the urgency of the situation, the Professional Ethics Front has urgently sought the intervention of the State Comptroller’s Office to ensure that the disturbing and serious findings are made public, as well as to immediately stop vaccinating young children and infants and prevent infant vaccination approval until the complete information on the safety of the vaccine in infants and children is fully investigated.
The Ministry of Health did not respond to questions sent to it – neither through the ministry’s spokespersons office nor through its COVID information headquarters.
