More and more COVID-19 vaccine injuries are starting to make their way into the medical journals. Several recent published case studies focus on skin diseases, and we are publishing a few of them here today as a service to the public, since the corporate media will seldom, if ever, cover this news.
Since there is already a plan in place to promote a monkeypox outbreak as one of the next big “pandemics” (see: Plandemic II Launched to Keep Pandemic Funds Flowing to Big Pharma: MonkeyPox), it is important to document these existing skin diseases that are already occurring following the mass COVID-19 vaccination campaigns.
It would not surprise me one bit if the criminal government “health” agencies such as the FDA and CDC started publishing photos like you see in these studies and label them all as some new variant of “monkeypox” which would cause fear and panic in the public, when in fact these skin conditions are more likely COVID-19 vaccine side effects.
Five cases of new-onset pemphigus following vaccinations against coronavirus disease 2019
The Journal of Dermatology, August 2022
Abstract
Pemphigus is a group of blistering disorders characterized by the formation of intraepithelial blisters in skin and mucous membranes induced by the binding of circulating autoantibodies to intercellular adhesion molecules. The pathogenesis is complex and not fully understood; however, genetic predisposition and various triggers are widely accepted as key factors in pemphigus development. A few cases of new-onset pemphigus following coronavirus disease 2019 (COVID-19) vaccination have already been published. The present paper reports a total of two cases of pemphigus foliaceous and three cases of pemphigus vulgaris that occurred following vaccinations against COVID-19, with anamnestic, clinical, and diagnostic data collection suggesting assumptions over a possible causal correlation.
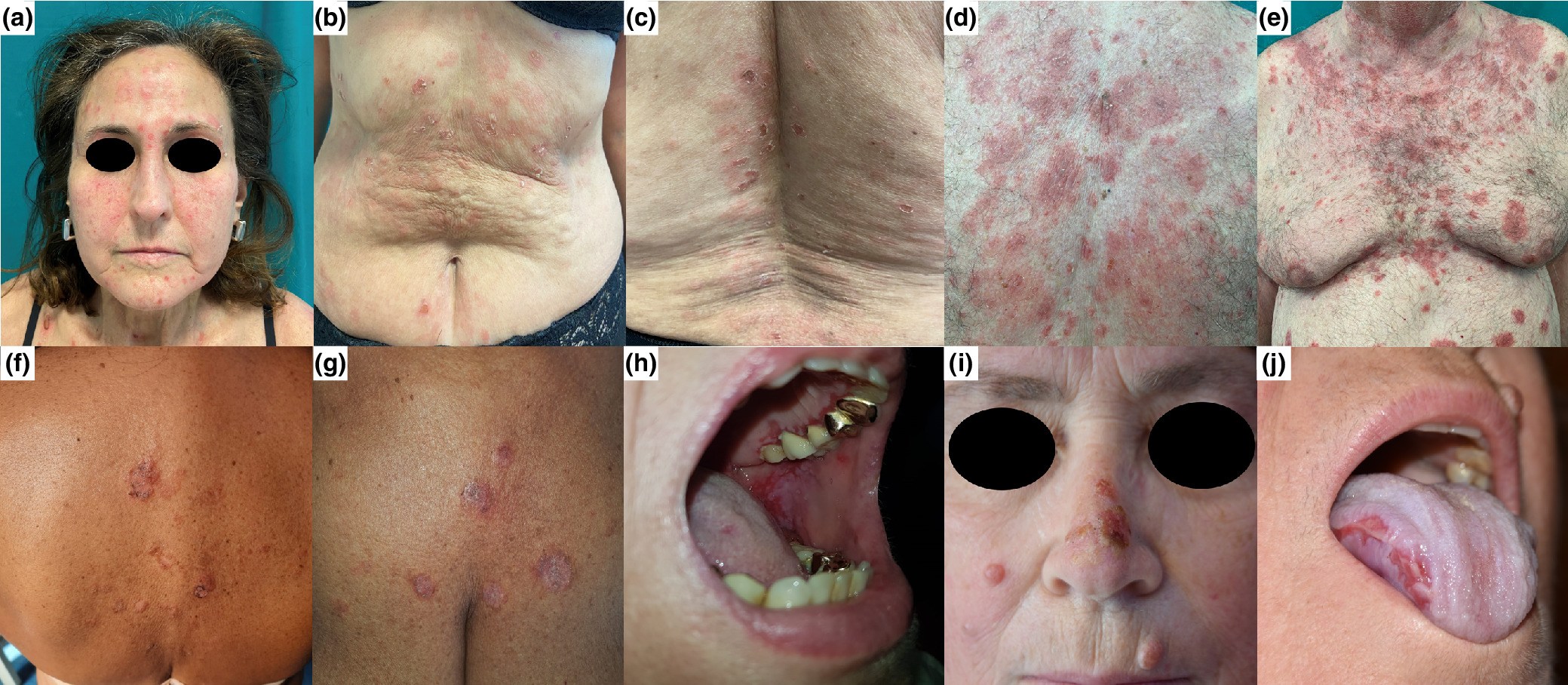
Clinical pictures of our cases. Case 1: scattered superficial blisters and erythematous patches with scaly erosions involving the face and lower trunk (a–c). Case 2: diffuse erythematous-squamous patches with scaly and crusted erosions involving the face and the trunk, following a seborrheic distribution (d, e). Case 3: a few erosive lesions, diffuse scales, and crusted erosions involving the trunk (f, g). Case 4: painful erosions on the gums and soft palate (h). Case 5: erosive lesions on the oral cavity, nose, right cheek, and abdomen (i, j).
Read the full study here.
Pemphigus foliaceus triggered after inactivated SARS-CoV-2 vaccine: Coincidence or causal link?
Dermatological Therapy, August 2022
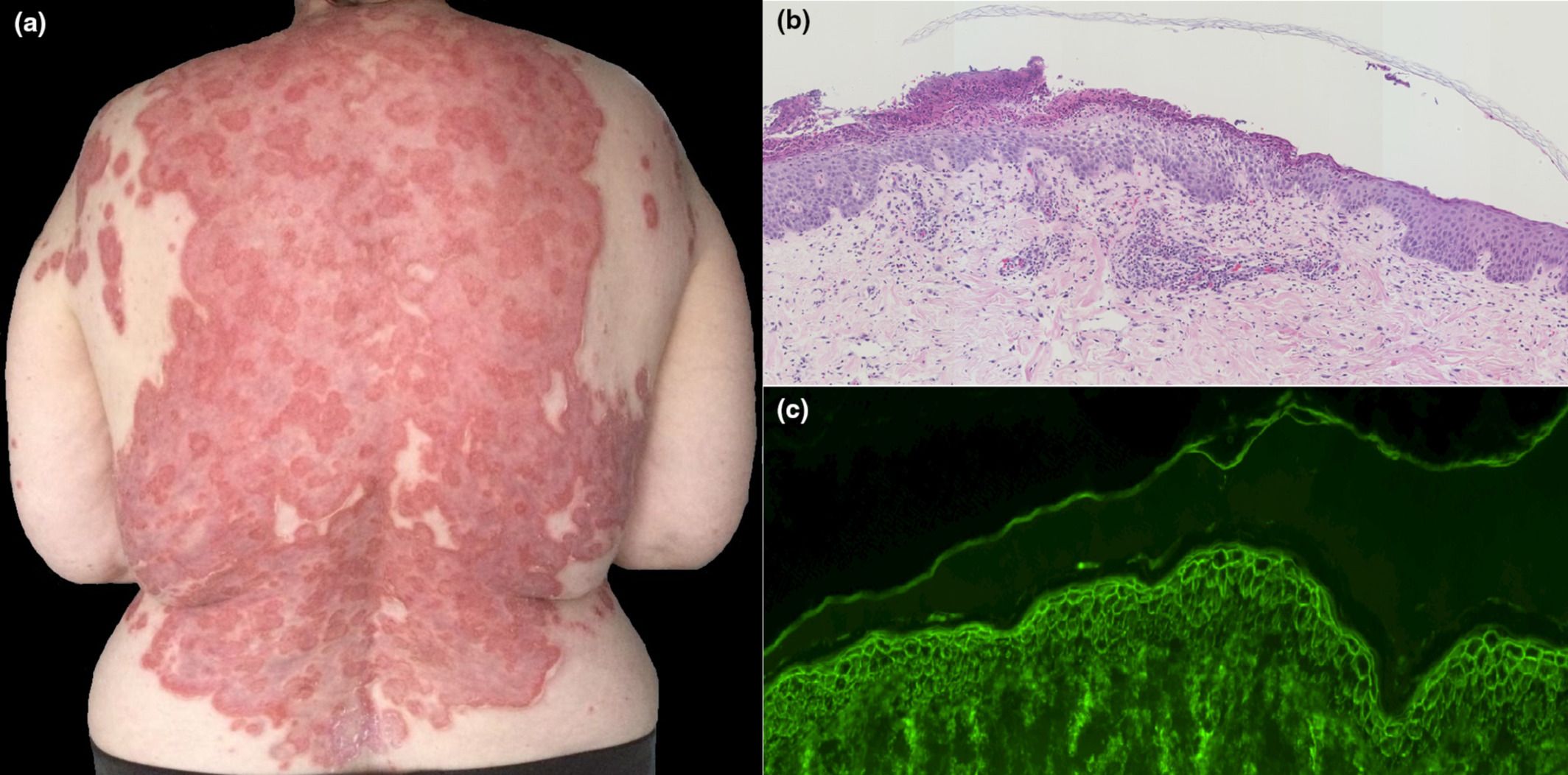
Pemphigus is a group of autoimmune blistering disorders associated with autoantibodies against the keratinocyte cell surface. Its exact cause is still unknown, but neoplasms, infections, medications, or vaccines are considered as possible triggering factors. Only one case of pemphigus vulgaris (PV) following vaccination with mRNA vaccine BNT162b2 has been reported.1 At the time of submission of the present report, this is the first case of Pemphigus foliaceus (PF) type triggered after inactivated SARS-CoV-2 vaccination.
(A) Clinical examination on admission showing scales and crusts with erythematous bases and superficial blistering all over the trunk. (B) Large erosions of the neck after extension of the superficial detachment. (C) Histopathology of lesion showing subcorneal loss of adhesion with acantholytic cells
Read the full study here.
New onset pemphigus foliaceus following AstraZeneca COVID-19 vaccination
Journal of the European Academy of Dermatology & Venereology – August 2022
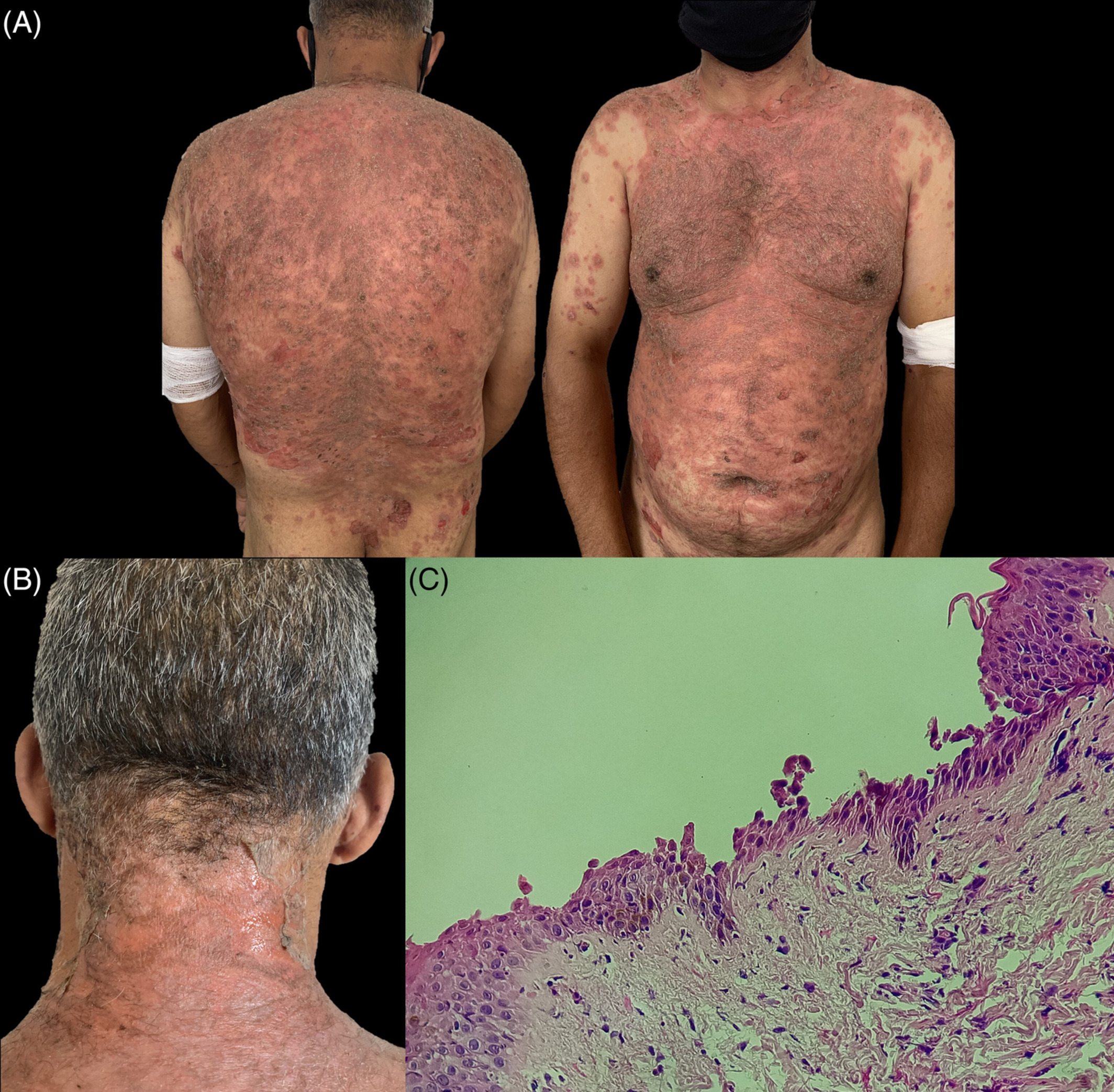
Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are a rare group of immunobullous disorders that can lead to high morbidity and mortality.1 Analogous to the other autoimmune diseases, pemphigus is closely related to the immune response, which is impacted by several factors including; polymorphism of the genes, family history of other autoimmune disorders including pemphigus, gender, ethnicity, geographical area and environmental factors.2, 3 Several triggers including different drugs and treatments, diseases, vaccines, nutrients, micronutrients, pregnancy and stress have all been implicated in the aetiology of the disease.4 Here, we present a case with PF whose disease developed after administration of the first dose of ChAdOx1 nCoV-19 (AstraZeneca) vaccination and was exacerbated following the second dose of this vaccine.
(a) Large erosive annular erythematous plaques on the back. (b) Histology of the skin shows subcorneal acantholysis and blister filled with neutrophils. The epidermis is infiltrated by a large number of neutrophils. H and E stain ×100 magnification. (c) Direct immunofluorescent staining shows intercellular IgG in the epidermis in a chicken wire-like pattern. IMF stain ×200 magnification.
Read the full study here.
Pemphigus vulgaris following second dose of mRNA-(Pfizer-BioNTech) COVID-19 vaccine
Dermatological Therapy, August 2022
Pemphigus vulgaris (PV) is an autoimmune, blistering dermatosis caused by autoantibodies to desmoglein (Dsg) 1 and (Dsg) 3 targeting keratinocyte desmosomes. The etiology of the disease remains unknown. There are many inciting factors for PV development, including infections, medications, ultra-violets, radiations, trauma, burns, and underlying neoplasms. A limited number of patients developed PV following vaccination.1-3
Only a few cases of PV following the COVID-19 vaccine were reported since the beginning of the pandemic. We report a case of PV occurring 1 week after the second dose of mRNA-Pfizer-BioNTech COVID-19 vaccine.
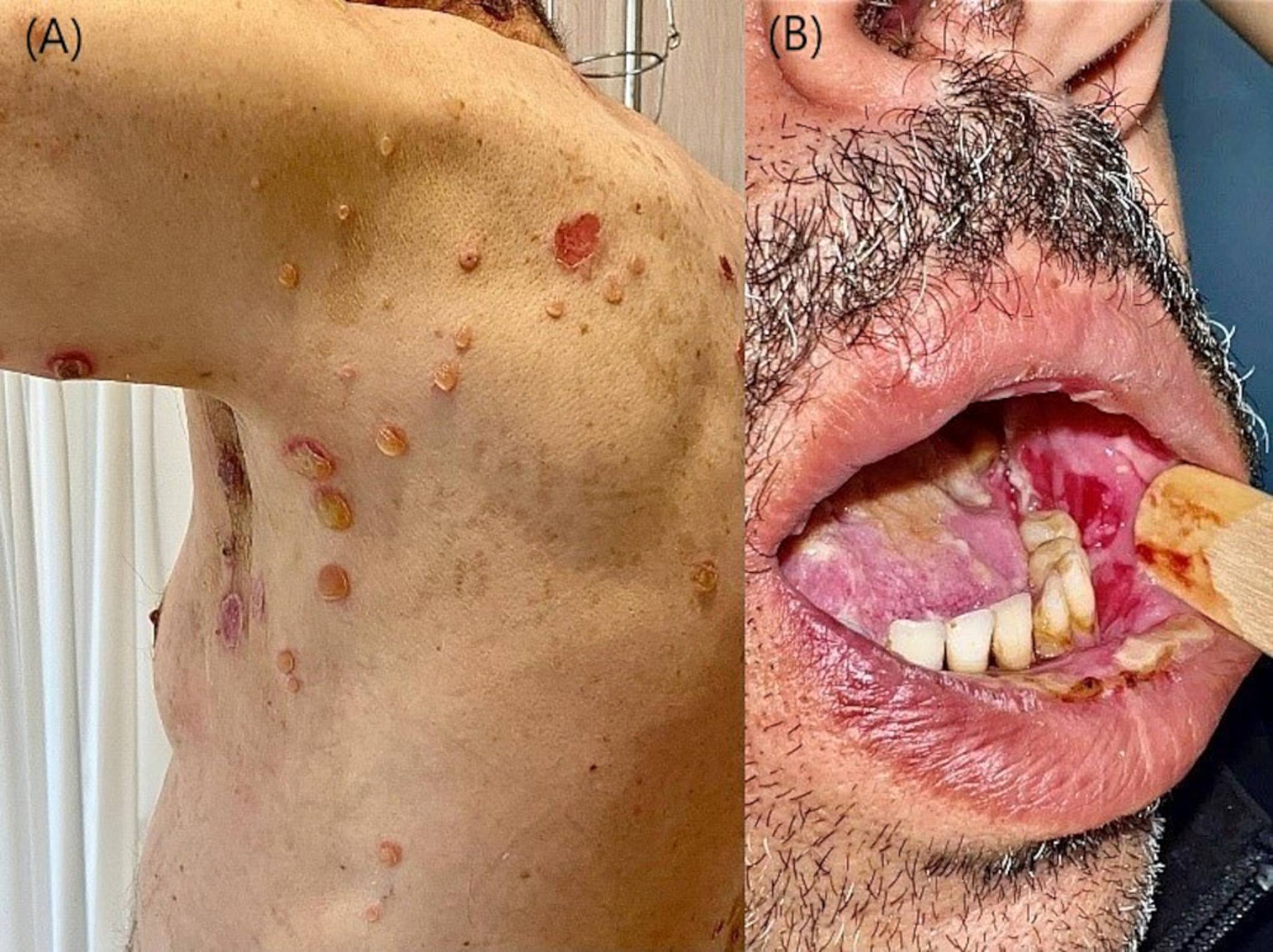
(A) Flaccid blisters on healthy skin with post-bullous erosions. (B) Post-bullous erosions of the oral mucosa
Read the full study here.
Pemphigus vulgaris with advanced hypopharyngeal and gastric cancer following SARS-CoV-2 vaccination
The Journal of Dermatology, August 2022
Pemphigus vulgaris (PV) is an autoimmune blistering disease that causes intraepidermal blisters on the skin and mucous membranes. Autoantibodies targeting desmoglein 1 and desmoglein 3, which are cell adhesion molecules connecting epidermal cells, play an essential role in the development of PV.1 It has been suggested that one mechanism for the production of autoantibodies is molecular mimicry, i.e. a phenomenon in which different molecules have similar structures. For example, if antigens of pathogenic microorganisms such as bacteria and viruses are in a molecular mimicry relationship with self-antigens, immune responses to infections may cause autoimmune diseases.2, 3 Autoimmune diseases caused by molecular mimicry could also be caused by vaccines. However, cases of PV occurring after vaccination are rare.
The mechanism by which PV is triggered by vaccination is unknown. Here, we report a case of PV with latent hypopharyngeal and gastric cancer that manifested after SARS-CoV-2 vaccination.
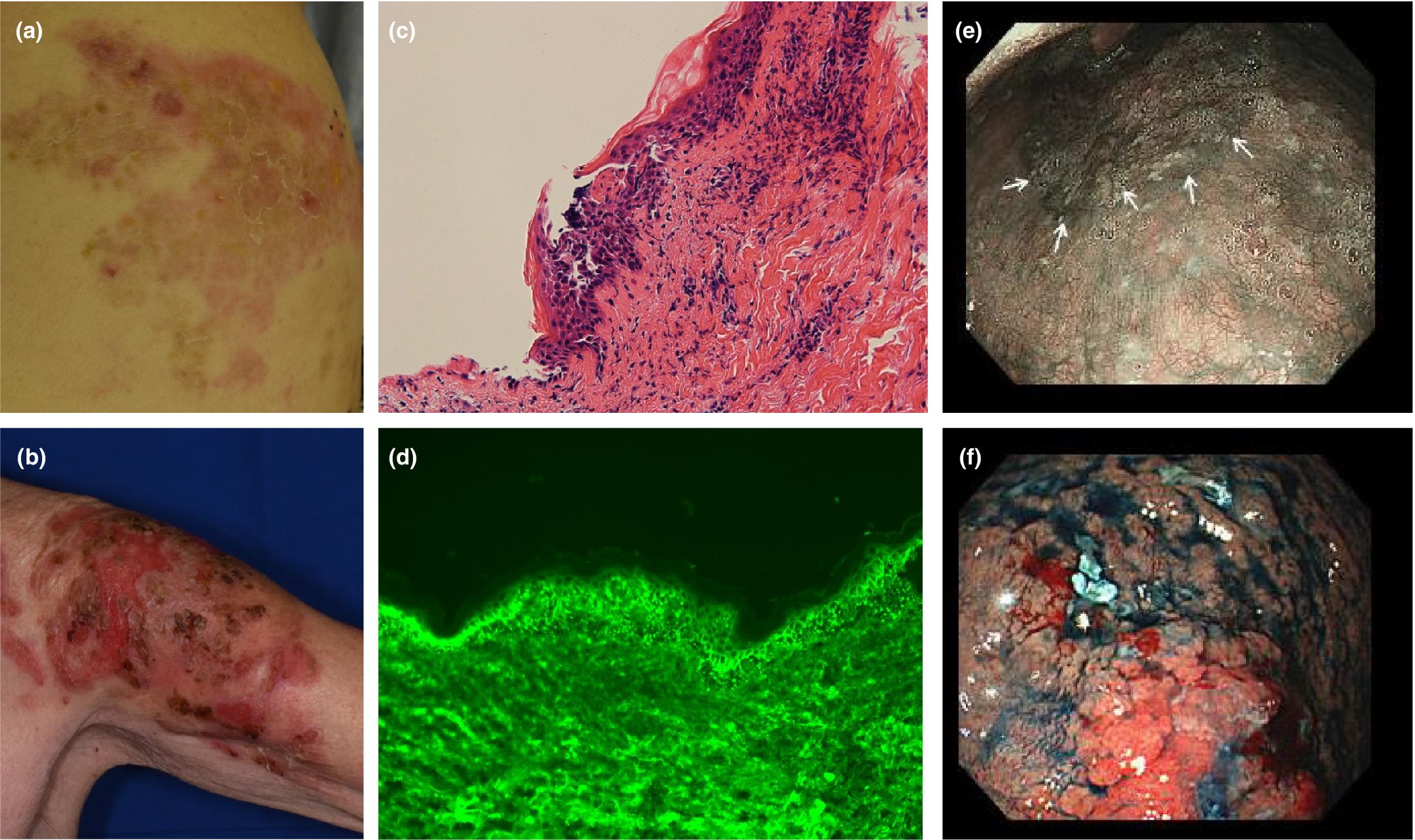
Skin symptoms, histopathological findings of skin blister lesions and gastrointestinal endoscopic findings. Flaccid bullae with erythema on the right lumbar region (a) and left upper arm (b) at the time of our hospital visit. The histopathological sections, stained with hematoxylin and eosin, revealed intraepidermal blisters (magnification × 200) (c). Direct immunofluorescent staining of tissue sections from blister lesions demonstrated IgG deposition on keratinocytes (magnification × 400) (d). Hypopharyngeal cancer (e) and gastric cancer (f). The white arrows indicate the margin of hypopharyngeal cancer.
Read the full study here.
Development of severe pemphigus vulgaris following ChAdOx1 nCoV-19 vaccination and review of literature
Journal of Cosmetic Dermatology – March 2022
Abstract
Vaccines are indeed a boon for tackling the present COVID-19 pandemic. In India, ChAdOx1 nCoV-19 (Covishield) is the most commonly used vaccine in the government vaccination program for adults more than 18 years of age. It is a recombinant vaccine developed by Oxford-Astra Zeneca and manufactured in India by Serum Institute of India (SSI). Here, we report a case of severe pemphigus vulgaris following the second dose of ChAdOx1 nCoV-19 vaccination in an adult male. The patient developed septicemia during the course of hospital stay, and he was managed with systemic steroids, parenteral antibiotics, and intravenous immunoglobulins (IVIg) along with proper wound care. Patient started improving within 1 month of therapy. This case is being reported in view of the rarity of pemphigus vulgaris following ChAdOx1 nCoV-19 vaccine.
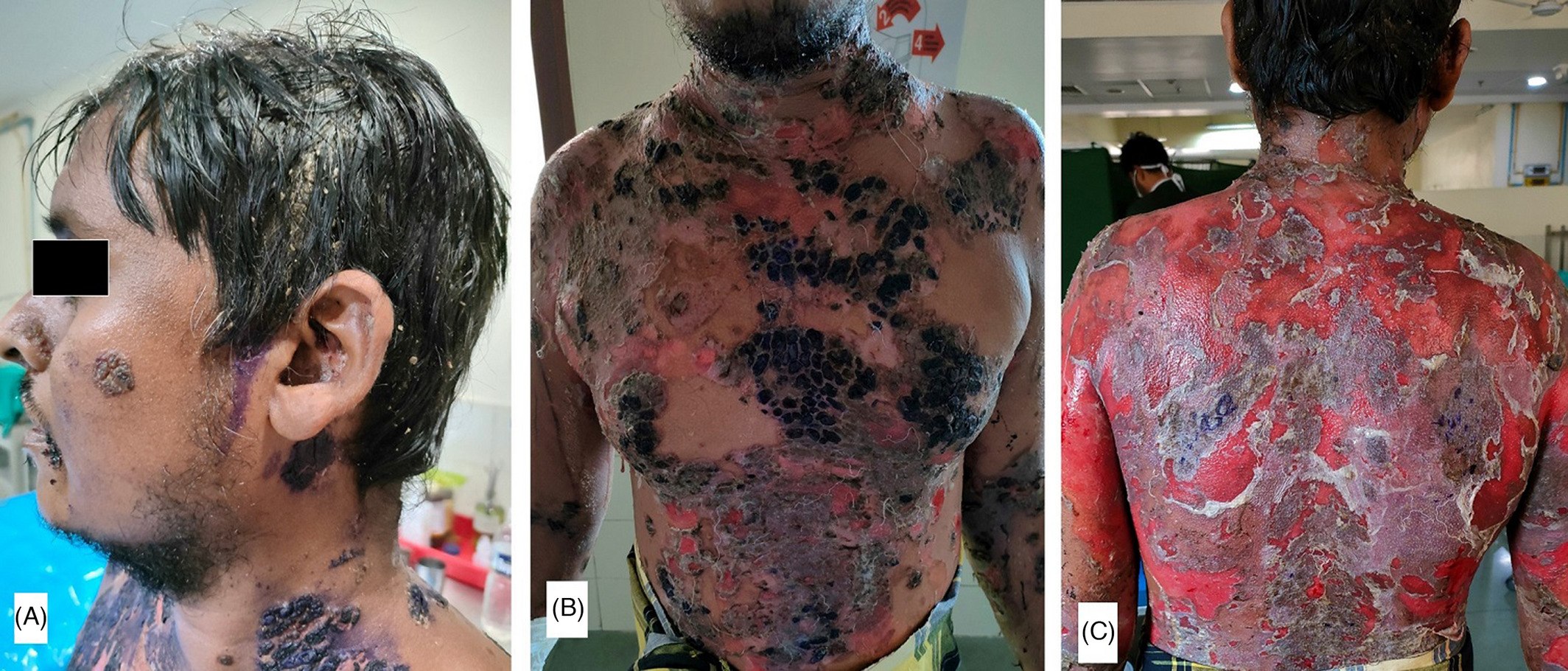
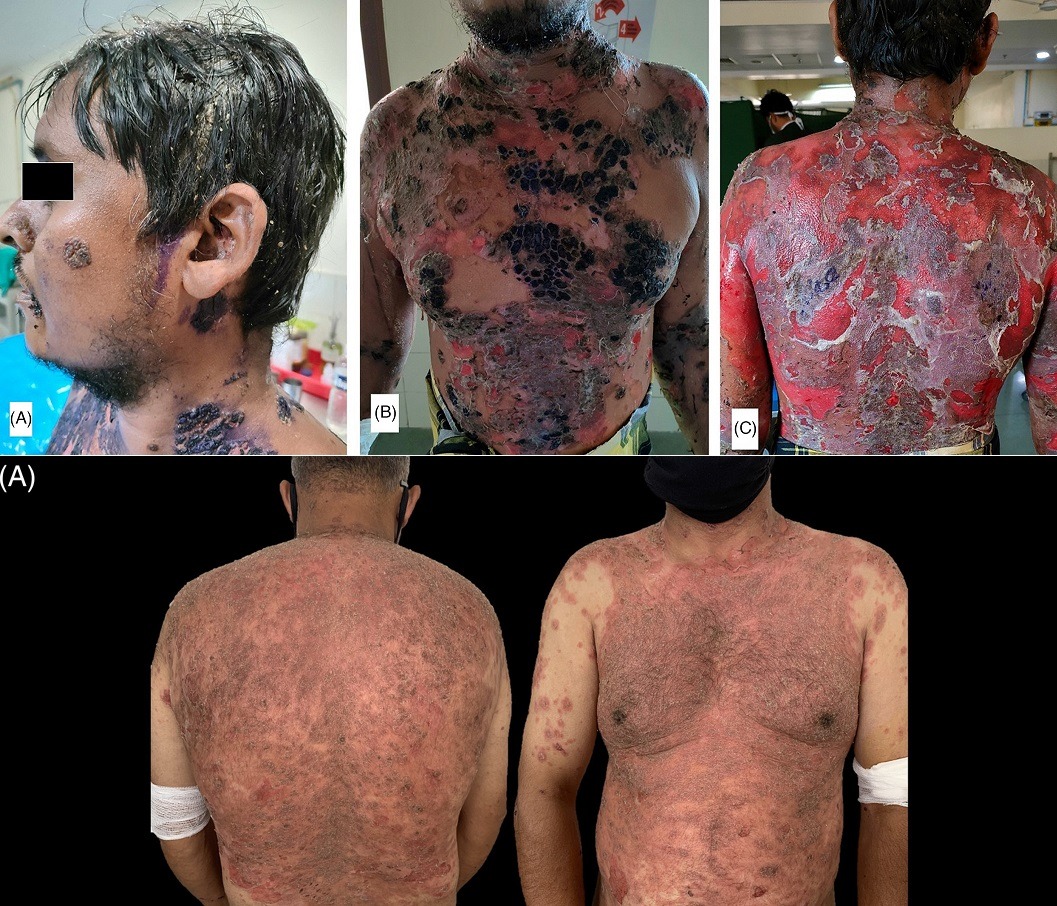
(A) Multiple crusted erosions over face and left ear along with matted hairs over scalp; (B) multiple crusted erosions with overlying gentian violet seen on chest and abdomen; (C): extensive erosions with some islands of normal skin present over whole back
Read the full study here.
