The study also finds evidence of ADE by the REGEN-COV/Ronapreve monoclonal antibodies
Researchers in Kyoto and Osaka have a new paper in Nature’s Scientific Reportson antibody-dependent enhancement of Corona infection by monoclonal antibodies and mRNA vaccination. Standard disclaimers: This is an in-vitro study; it doesn’t look at clinical outcomes; things that seem bad in a cell culture might not mean very much in real life.
Still, the findings aren’t fantastic.
The authors look at the monoclonal antibodies known as casirivimab and imdevimab, sold in combination as REGEN-COV or Ronapreve, among other names. They find that, at the right antibody concentrations, there is enhancing activity. That is, if you infect a bunch of cells with SARS-2 (wild-type), and then introduce the monoclonal antibodies at very low concentrations, it’s the same as having no monoclonal antibodies at all. If you increase the concentration slightly, SARS-2 replication is actually enhanced, until you increase it still more beyond this narrow window and replication is inhibited.
This is what that looks like:
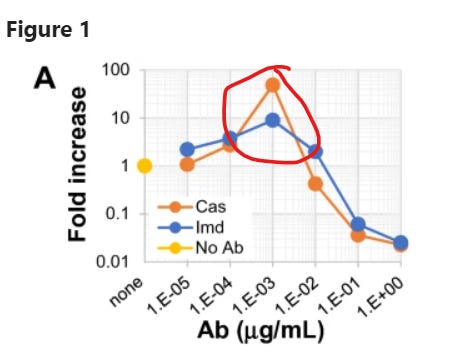
From left to right on the x-axis, the monoclonal antibody concentration grows. The y-axis measures how much more virus there is in the antibody-treated samples, than in the no-antibody controls. Beware the log scale; 1 here is the no-antibody baseline, above 1 is enhancement, below 1 is neutralisation. In red, I’ve circled the narrow window of dilution where virus replication is enhanced.
You need effective antibodies for antibody-dependent enhancement, and because Omicron is pretty good at evading casirivimab and imdevimab, it doesn’t get much of a boost from them at any concentration.
The authors note that their findings “raise the possibility that SARS-CoV-2 mRNA vaccines targeting the S-protein also induce ADE-causing [antibodies] as well as neutralizing [antibodies],” so they repeated the same basic experiment, this time not with monoclonal antibodies, but with sera from a few vaccinated volunteers.
First they tested how well the vaccinated sera performed against the wild-type SARS-2:
Neutralizing activity was not detected in serum collected on day 27 after the first vaccination, but was detected at the highest concentration (1/100 dilution) of serum collected on days 20 and 52 after the second vaccination … Simultaneously, obvious ADE activity was also detected at a diluted concentration (1/10,000 dilution) of serum. Importantly, serum collected on day 98 after the second vaccination exhibited no neutralizing activity at all under the serum dilutions examined, but maintained clear ADE activity.
Here’s what the graph looks like:
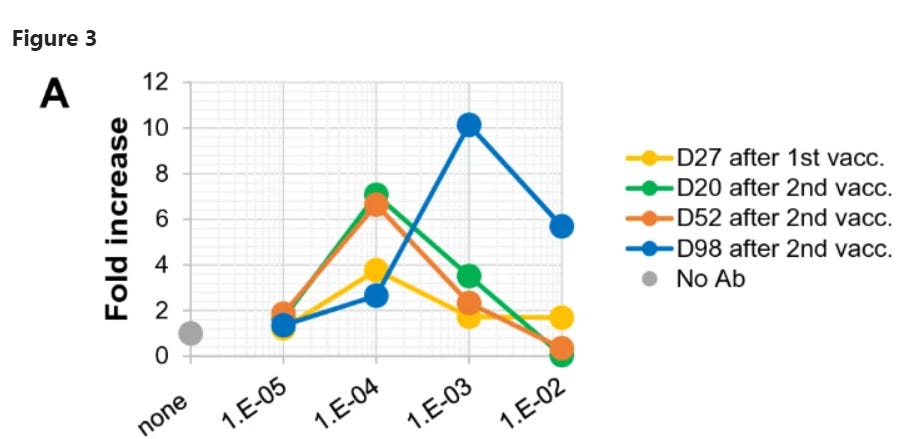
This is serum from just one volunteer, “HC2.” For some reason, by the time he was 98 days out from vaccination, this volunteer’s serum did nothing but enhance virus replication in vitro. Perhaps disturbed by this result, the researchers grabbed a few other volunteers to see if their sera also enhanced viral replication at the 98-day mark. The answer, happily, is “no”:

They exhibited some weak ADE effects when more diluted, but became neutralising at higher concentrations. If you wait even longer, though, things don’t look so rosy anymore. At 175 days post-vaccination, our unfortunate volunteer HC2 was no longer the only one whose serum seemed to enhance viral replication. Another volunteer, HC4, had suddenly joined him:
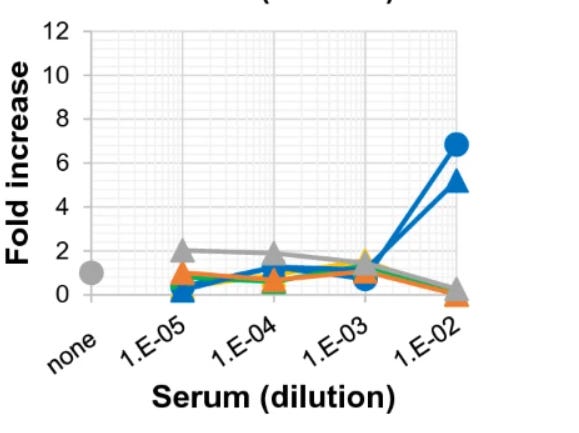
Note that the pattern has changed. Closer to vaccination, there was a window of dilution where ADE occurred. At very low serum concentrations, you might as well have unvaccinated serum; at high concentrations, the antibodies neutralised the virus. Only in the middle did the sera enhance infection. Very far out from vaccination, that’s no longer the case. Now, it’s the highest serum concentrations where ADE happens.
The authors:
Taken together, these results demonstrate that after vaccinations, neutralizing Abs are induced and persist for a long time in some individuals, but ADE-causing Abs also exist from the early stage and persist for a longer period than do neutralizing Abs in some individuals.
All of this is to some degree ancient history; wild-type SARS-2 is dead. How do the vaccine-elicited antibodies fare against Omicron? Of their six volunteers, one – HC6, in grey – had serum that clearly enhanced Omicron replication at high concentration:
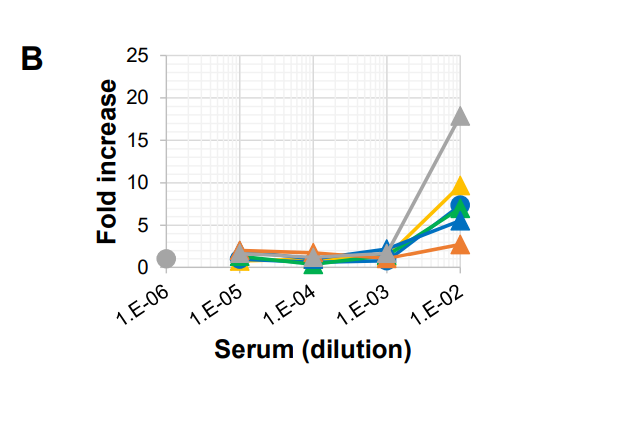
(The other samples in this graph might also seem to indicate some smaller measure of ADE, but they’re not significantly different from the effects of the pre-vaccination controls provided by the same volunteers.)
The authors again: “These results suggest that the rapid spread of Omicron around the world may in part result from the lack of cross-neutralization against Omicron and some ADE activity of sera after vaccination.”
Remember how everywhere mass vaccination occurs, we tend to see higher frequencies of infection? Remember how, in the deeply unadjusted and unreliable UKHSA vaccine efficacy statistics from the start of the year, the double-vaccinated appeared to experience higher rates of infection and death than the unvaccinated? Crazy conspiracy theorists might be forgiven for wondering if these are the effects of antibody-enhanced virus replication in the lungs of people more than six months out from their second jab.
Again: We don’t know whether this effect has any clinical manifestation; the human immune system is more than antibodies, and it’s possible that people with replication-enhancing sera don’t have measurably different experiences with SARS-2 than people with neutralising sera. It’s well within the realm of possibility, though, that as a result of these ADE effects, a nontrivial number of vaccinated people shed much more virus than their unvaccinated counterparts.
Source – https://www.eugyppius.com/p/japanese-study-finds-in-vitro-evidence
