One of the accepted side-effects of the Covid vaccines is increased risk of myocarditis. This risk was first identified in a leaked report produced for the Israeli Government, which was followed by several months of the denial of any link by the vaccine manufacturers and various governments until eventually the evidence for this side-effect became overwhelming and it was added to the growing list of official side-effects.
Of course, once a potential risk of myocarditis was identified there were some attempts by epidemiologists to sift though available data to try to identify the impact of this risk on the vaccinated population. The most recent paper to study this side-effect appeared in late August out of the Nuffield Department of Medicine in Oxford Radcliffe hospital by a team led by Julia Hippisley-Cox. This paper purports to show that the risk of myocarditis following vaccination is much lower than the risk following infection with Covid and that the risk of myocarditis following infection in the vaccinated is much lower than the risk in the unvaccinated. Thus the paper implies that the vaccines are appropriate to use even with this ‘rare’ side-effect. However, there are some complexities in the paper that warrant further attention.
My main concern with the Hippisley-Cox paper is that it is based on the results of a self-control study. With this methodology, the baseline data (i.e., the rate of myocarditis that you’d expect without the vaccines) are gathered from the same individuals as took the vaccine, only outside of the ‘risk window’ that is said to be associated with the vaccines. This methodology is well established and has many advantages, the most important of which being that so long as the study is well designed there is only a low risk of bias because the same group of individuals acts as its own control. The ‘so long as the study is well designed’ in the previous sentence is, however, crucial: pivotal in the design assumption is that the risk window contains all of the additional risk and that the risk returns to baseline outside of this window. If this condition doesn’t obtain then the incidence in the ‘control group’ will be elevated and this will reduce the apparent excess in the ‘treatment group’.
For the Hippisley-Cox paper the main assumption is spelt out in the methods section (emphasis added):
We defined the exposure risk intervals as the following prespecified time periods: 0, 1-7, 8-14, 15-21 and 22-28 days after each exposure date, under the assumption that the adverse events under consideration are unlikely to be related to exposure later than 28 days after exposure.
The pertinent question, then, is: is this assumption correct? Let’s have a look at the risks identified in the paper for the 28 days following the first dose of vaccine, split into four week periods.
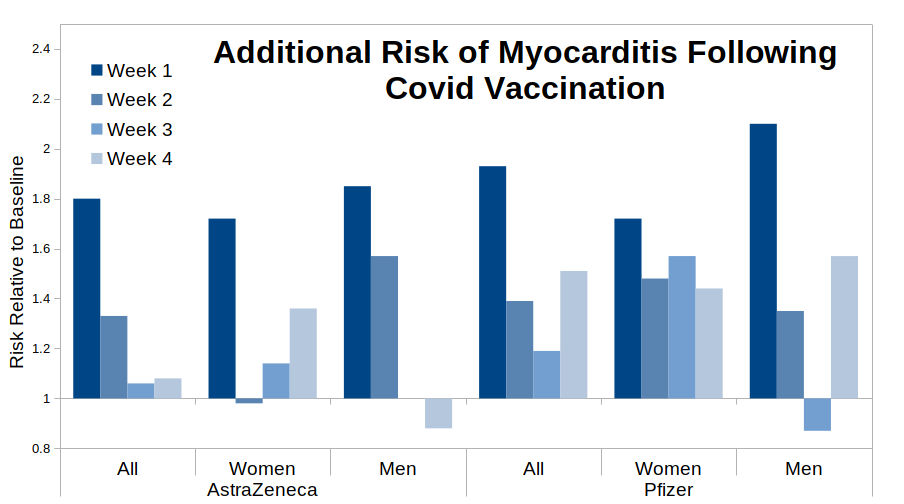
To be clear – if the paper’s assumption that all of the vaccine risk occurs within the four weeks following vaccination then we should see a gradually falling risk over the four week period, preferably to a relative risk of 1.0 (no additional risk) by week 4. Arguably we do see this pattern for the “All, AstraZeneca” data – it appears that most of the risk is in the first week following vaccination, the risk drops away to a halfway point by week two and falls to around 1.0 for weeks three and four. However, the data for the Pfizer vaccine are far less clear – I suggest that it looks more like the risk starts to fall away (e.g. see the “all” and “men” bar-charts) but then bounces back for week four post-vaccination. The fact that the ‘bounce’ appears for women given the AstraZeneca vaccine even suggests that this might be a common factor for all vaccines, but the pattern appears strongest for the Pfizer data. Further investigation into the Pfizer vaccine specifically suggests that this pattern is fairly reliable, with only younger women not showing the bounce.
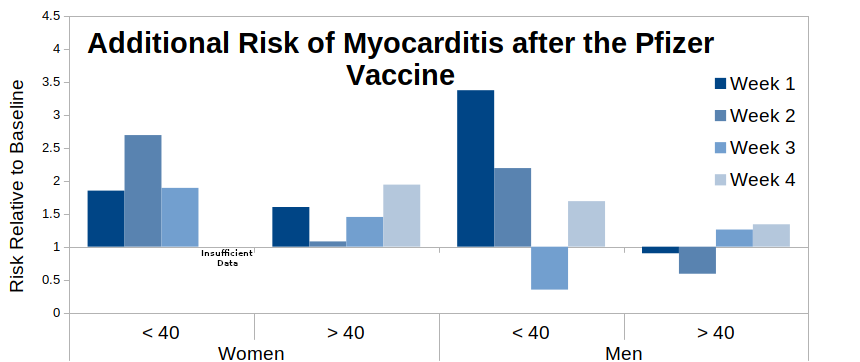
What I’d expect to see in a paper using a self-control method would be to have a ‘sensitivity analysis’ in the supplementary data, showing that the risk did actually return to baseline by week five (or however long it took to return to baseline), however there are no such data. Thus the authors expect the reader to believe that the above bar-charts all miraculous return to baseline for the fifth week – I believe that their data suggest that this is unlikely and that the risk of myocarditis is maintained beyond 28 days post vaccination, at least for the Pfizer vaccine. The situation is less clear for the AstraZeneca vaccine, and while the data for the Moderna vaccine (not shown) suggest that it is associated with a much higher risk of myocarditis, the relatively low numbers vaccinated with Moderna make it difficult to see if it also suffers from the ‘bounce’ in myocarditis risk.
It is important to note that if the risk is maintained beyond 28 days then there are two consequences:
- The risk is higher than the paper suggests, as it persists over a longer period of time;
- The baseline data will have some post-vaccine myocarditis mixed in with them; this will elevate the baseline risk and thus make the post-vaccine risk appear lower.
I’m always disappointed when self-control studies make this mistake. In science it is acceptable to have assumptions in a method, but it is necessary to test those assumptions and ensure that they are valid. In this case they appear to have declared that it is necessarily true that all additional myocarditis cases lie within the 28 day post vaccination period, and that it is not necessary to even consider that the declaration is false (I suggest that this is how religions work, not science).
One point that is encouraging about the data from the Hippisley-Cox myocarditis paper is that the data suggest that the bounce in myocarditis risk in the 21-28 day period after the first dose of vaccine doesn’t appear for the second and third doses. Of course, it might be that a bounce occurs after a longer delay for subsequent doses – but without additional data and with only sparse information on potential mechanisms that can only be a guess. Nevertheless, there is a suggestion that risks in the first seven days after vaccination are higher with the second and possibly third doses – this is consistent with the conclusions of other recent papers on myocarditis risk from Nordic countries and France, although these other papers do suggest that the risk after the second dose is higher than indicated by the Hippisley-Cox paper.
One potential mechanism that might explain the data seen is that following vaccination there is a two stage process – firstly a myocarditis related to direct damage to the myocardium from the fast-acting innate immune system, followed a few weeks later by (additional) damage caused by an adaptive (auto-)immune response (e.g. see this study). This potential mechanism would also explain the lack of a bounce in later doses, as the body would be primed to produce the adaptive (auto-)immune response rapidly following the appearance of new spike proteins.
There’s another aspect to the paper that is troubling – this lies in a small sentence also tucked away in the method (emphasis added):
Incidence rate ratios (IRR), the relative rate of hospital admissions or deaths caused by myocarditis in exposure risk periods relative to baseline periods, and their 95% CIs were estimated by the self-controlled case series model adjusted for calendar time.
The adjustment for calendar time means that they took into consideration the risk change given the time of year. Again, this is reasonable as it might be expected that myocarditis risk varies with the season – indeed, there is some evidence that the background rate of myocarditis is greater in the winter, although other studies suggest that myocarditis risk doesn’t vary much by season (science is inconsistent like this).
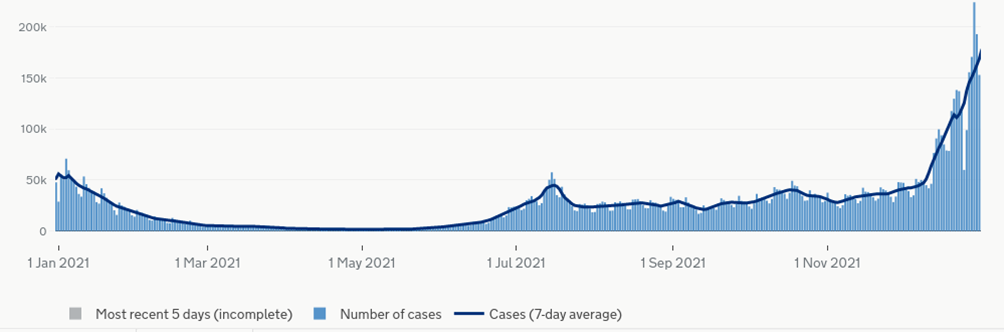
We can estimate the magnitude of this adjustment using the data on the increase in the risk of myocarditis after infection with COVID-19 by vaccination status. In the study all participants were vaccinated, thus the majority of infections before vaccination will have occurred early in the study and those post-vaccination will have occurred later. Luckily, the infection risk during the study was split into two periods – a winter Covid wave (Alpha variant), a period of low Covid incidence for several months and then a sustained period of moderate risk from mid-summer (Delta variant). This is illustrated in the chart of Covid infections for England during 2021.
In the paper the authors provide information on the relative increase in risk of myocarditis for the pre-vaccine and post-vaccine Covid infections, the number of cases of myocarditis that occurred and also the total number of infections pre- and post-vaccination. Using these data we can estimate the background rate of myocarditis used in the paper for the pre- and post-vaccination periods (mainly Jan/Feb and Jul-Dec respectively).

Hmm. This is troubling – we’ve got an increase in background myocarditis rate for a period that includes the summer (0.45), compared with the background rate for winter (0.34). This goes against the evidence that myocarditis risk is either independent of season or has a greater incidence during winter.
I suggest that what we could be seeing here is the impact of an extended period of increased risk of myocarditis post-vaccination (as discussed earlier in this post), which has increased the baseline used in the paper for the post-vaccination period. These data suggest an increase in risk of around 30%, which seems plausible given the data given for the 28-day post vaccination period.
This isn’t an undeniable proof that the paper is underestimating the risks of myocarditis after vaccination, but it adds to the red-flags that suggest that its methodology isn’t correctly identifying the increased risk.
There’s one more aspect to the paper. The authors claim that the vaccines reduce the risk of post-infection myocarditis to about half that seen in the unvaccinated. Is this merely an artefact caused by a changing baseline – which would explain much of the difference in risk for the first week after infection? Note that the vaccines don’t appear to reduce the risk of infection with Covid, thus if there is no reduction in the risk of myocarditis post infection in the vaccinated then there are only increased risks of myocarditis following vaccination without any ‘benefit’ (considering only myocarditis risk).
Furthermore, the pre-vaccination infections were with Alpha variant, whereas the post-vaccination were nearly all Delta-variant. How much of the reduction in risk seen ‘post-vaccination’ were caused by Delta variant having a different risk of myocarditis? Indeed, the paper considers that the varying risk of hospitalisation with myocarditis is purely related to vaccination or infection with Covid – were there other factors that resulted in a change in the probability of hospitalisation? Perhaps the public became more aware of the symptoms of myocarditis and being more likely to seek healthcare, or those infected with Covid were more likely to be hospitalised with Covid and had myocarditis diagnosed during the admissions process. And, of course, let’s not forget the cardiac risks arising from climate change, sitting incorrectly and drinking the wrong types of carbonated beverage that have suddenly appeared over the last 18 months.
It is worth pointing out that the very low risks identified in the paper were for hospitalisation because of myocarditis. The paper gives no information on the number of cases of myocarditis that might have warranted hospitalisation but that were ignored by the individual concerned, or for myocarditis with only mild symptoms. I note that a paper published recently on the topic of indicators of post-vaccine cardiac damage in young males suggests that this risk might be rather high. Unfortunately, there’s little information on the potential longer term consequences of this vaccine-associated myocarditis or other cardiac damage (of whatever clinical significance).
It also needs to be added that the paper only discusses one particular risk associated with the vaccines; other risks have been identified, and there may be other health impacts over the longer term that are currently being ignored. The paper is also deficient in that it does not consider the role of natural infection in potentially lowering the risk of post-infection myocarditis (this is important given that nearly everyone in the U.K. will have now had at least one Covid infection).
What disappoints me most of all is that we have ended up with a plethora of observational studies, each of which has attempted to identify ‘the scientific truth’ with incomplete and messy data. The reality is that we had a set of under-tested vaccines that were unleashed onto many multiple millions of individuals without any attempts to undertake large prospective matched cohort trials, even though such trials are normal on the release of a new medical product, there have been vast amounts of money thrown at Covid-related projects and after the initial rollout to the most very vulnerable there was sufficient time to organise such trials (there aren’t even signs of such a study on the rollout of the bivalent Covid vaccines, which appear to be somewhat under-tested). Indeed, we’re now in the era of ‘big data’ where such studies could readily have been integrated with real-time GP and hospital data to get a good and timely understanding of risks as they emerged (rather than the rather pathetic Yellow Card system).
